Active in the nineteenth century, Niels Bohr (1885 – 1962) was a Nobel Prize winning Danish physicist who made several important contributions to science including his revolutionary model of the atom, complementarity principle, Copenhagen interpretation of quantum mechanics and the liquid drop model explanation of nuclear fission. Know more about his discoveries, theories and other accomplishments as a scientist by studying his 10 major contributions to science.
#1 HE DISCOVERED THE BOHR–VAN LEEUWEN THEOREM
Niels Bohr worked on his PhD thesis during 1910 – 1911, in which he discovered the Bohr–van Leeuwen theorem. Later rediscovered by Dutch physicist Hendrika Johanna van Leeuwen, the theorem states that when statistical mechanics and classical mechanics are applied consistently, the thermal average of magnetization is always zero. The importance of Bohr’s discovery is that classical physics does not allow for such things as para-magnetism, diamagnetism and ferromagnetism, and thus quantum physics is needed to explain these magnetic events. The Bohr–van Leeuwen theorem is useful in several applications including plasma physics. Electro-mechanics and electrical engineering also practically benefit from it.

#2 He INTRODUCED THE BOHR MODEL OF THE ATOM IN 1913
In 1911, British physicist Ernest Rutherford formulated the Rutherford model of the atom by which an atom contained a very small charged nucleus orbited by low-mass electrons. Niels Bohr applied Max Planck’s quantum theory to the Rutherford model to come up with his famous Bohr model of the atom. The structure of the Bohr model was similar to that of a solar system with electrons orbiting the positively charged atomic nucleus in fixed orbits. Although the Bohr model has been superseded, its underlying principles remain valid and due to its simplicity, it is still taught in classes to introduce students to quantum mechanics.
#3 HIS APPLICATION OF QUANTUM CONCEPT TO THE ATOMIC MODEL WAS REVOLUTIONARY
The major improvement of Bohr’s model to the Rutherford model was the quantum physical interpretation of it, which remains sound. In classical physics, electrons could have any energy while quantum physics restricts the energy of a system to certain discrete values. In the Bohr model electrons can only occupy specific orbits of fixed energy at set distances from the nucleus. Most importantly, while jumping from one allowed orbit to another, they absorbed or emitted electromagnetic radiation. Bohr model thus offered the explanation of how matter could absorb and emit light, a scientific puzzle till then.
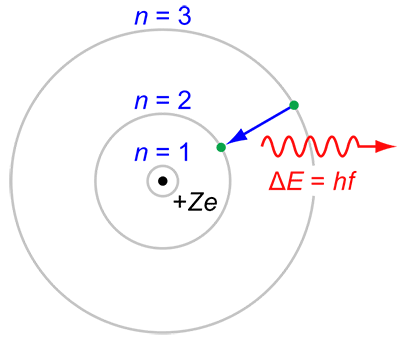
#4 HE EXPLAINED THE STRUCTURE OF THE PERIODIC TABLE THROUGH HIS ATOMIC THEORY
Niels Bohr applied his atomic theory to the periodic table of elements. He showed that chemical properties of an element resulted mainly from the behavior of valence electrons, the electrons occupying the highest stable orbit. This was an important step in the creation of the field of quantum chemistry. Also, Bohr predicted that the undiscovered atomic element 72 would resemble zirconium. His prediction was correct and Hafnium was discovered in 1923. The element took its name from Hafnia, the Latin version of Copenhagen, the home town of Bohr.

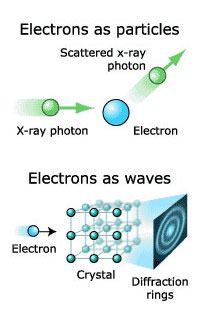
#5 HE FORMULATED THE COMPLEMENTARITY PRINCIPLE
In 1927, Niels Bohr for the first time formulated the principle of complementarity, which states that on the atomic level a physical phenomenon expresses itself differently depending on the experimental setup used to observe it. Thus, light appears sometimes as waves and sometimes as particles. Examples of complementary properties thus include wave-particle duality. Complementarity, by which complementary properties of an object can’t be measured at the same time, formed the basis of early quantum theory and is both a theoretical and an experimental result.
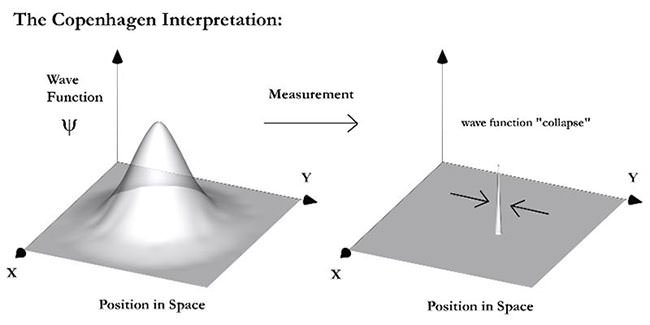
#6 He Co-DEVISED THE COPENHAGEN INTERPRETATION
Between 1925 and 1927, three leading twentieth century physicists – Niels Bohr, Werner Heisenberg and Wolfgang Pauli, devised the Copenhagen interpretation of quantum mechanics. By it, systems at atomic level don’t have definite properties prior to being measured. The act of measurement affects the system and causes it to select one of the various possible values after measurement. This feature is known as wavefunction collapse. The Copenhagen interpretation still provides a conceptual basis for quantum mechanics and remains among the most prominent interpretations of the theory.
#7 HE CAME UP WITH THE COMPOUND-NUCLEUS MODEL IN 1936
In 1936, Niels Bohr formulated the Compound-nucleus model. It explained nuclear reactions as a two-stage process. First the bombarding particle becomes an integral part of a new, highly excited, unstable nucleus, called a compound nucleus. The compound nucleus then loses its energy in different ways, such as losing a neutron or emitting gamma rays. The compound-nucleus model is successful in explaining nuclear reactions induced by relatively low-energy bombarding particles. The model was the most prominent description of the process for two decade, till it was improved upon by Niels’ son Aage Bohr.
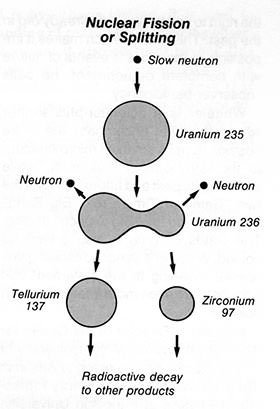
#8 HE EXPLAINED NUCLEAR FISSION THROUGH HIS LIQUID DROP MODEL
In 1939, Bohr collaborated with American theoretical physicist John Archibald Wheeler to develop the liquid drop model in nuclear physics to explain the mechanism of fission. In the model, the structure of nucleus was like a liquid drop of incomprehensible liquid. Like a drop could be deformed from its basic spherical shape to form two new drops, a large atomic nucleus, like uranium, could fall apart to form two new atomic nuclei. The liquid drop model was a ground-breaking work to explain nuclear fission theoretically.
#9 HIS DEBATES WITH EINSTEIN BROUGHT QUANTUM MECHANICS IN FOCUS
Albert Einstein and Niels Bohr were involved in a series of public debates about quantum mechanics. These debates are considered important to the philosophy of science. They not only represented one of the highest points of scientific research in the first half of the twentieth century but also brought to focus quantum non-locality, which is absolutely essential in our modern understanding of the physical world. It is generally believed that Bohr proved victorious in his defense of quantum theory.

#10 HE MADE A Key CONTRIBUTION IN DEVELOPMENT OF QUANTUM MECHANICS
Niels Bohr was one of the most prominent physicists of the 20th century. He made essential contribution to our understanding of the atomic structure, for which he was awarded the Nobel Prize in Physics in 1922. He also played an instrumental role in the establishment and development of quantum mechanics, whose applications include light-emitting diodes, the laser, the transistor, semiconductors such as the microprocessor, medical and research imaging, and electron microscopy. It provides explanation for many biological and physical phenomena too. Niels Bohr was also part of the Manhattan Project to produce the first nuclear weapons. However he was an outspoken advocate for the peaceful application of atomic physics and was awarded the first ever Atoms for Peace Award in 1957.

THE DANISH RESCUE OF JEWS TO SWEDEN
Apart from his immense contribution to science, Niels Bohr also played a part in the famous Danish Rescue of Jews to Sweden. After German occupation of Denmark, Bohr came to know that he was in danger of being arrested as his mother was a Jew. The Danish resistance helped him and his family to escape by boat to Sweden on September 29, 1943. Though there was an arrangement for Bohr to leave immediately for America to help in the Manhattan Project to produce nuclear weapons, Bohr refused to leave Sweden till he had met Swedish King Gustaf V. The next day, during their meeting, he persuaded Gustaf V to make public Sweden’s willingness to provide asylum to Jewish refugees. This was followed by mass rescue of Danish Jews with evacuation of 7,220 of Denmark’s 7,800 Jews to Sweden. Though its credit goes to Sweden and the Danish resistance, Bohr did play his part.

