Michael Faraday (1791 – 1867) was an English scientist who made an immense contribution to physics and chemistry especially in the fields of electromagnetism and electrochemistry. Among his inventions are the first electric motor and the first electromagnetic generator. His discoveries include the principles underlying electromagnetic induction, diamagnetism and electrolysis. He also discovered the principle of electrostatic shielding to invent the Faraday Cage and found the first experimental evidence that linked electromagnetism and light through a phenomenon known as Faraday Effect. Michael Faraday is considered one of the most influential scientists in history. Here are his 10 major contributions to science including his inventions, discoveries and monumental work in electromagnetism.
#1 HE INVENTED THE FIRST ELECTRIC MOTOR
In 1820, Danish physicist and chemist Hans Christian Orsted discovered that flow of electric current through a wire produced a magnetic field. His discovery of electromagnetism initiated intensive research in the field. Michael Faraday was the first to understand that this discovery meant that if a magnetic pole could be isolated, it ought to move constantly in a circle around a current-carrying wire. In 1822, Faraday invented the first electric motor, a simple device that could convert electrical energy into mechanical energy. Known as a homopolar motor, his invention was useful only for demonstrative purposes. However, it was the first step in the evolution of the immensely useful electric motor.
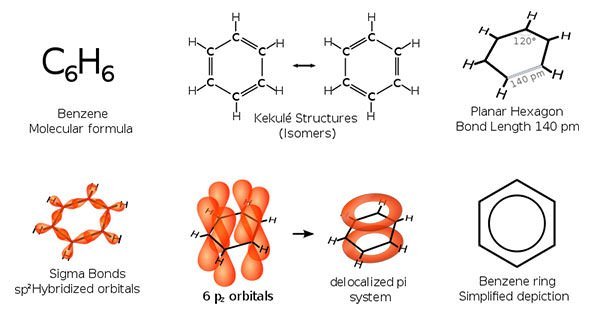
#2 HE DISCOVERED BENZENE
Michael Faraday achieved his early renown as a chemist. He made many important contributions to chemistry. In 1820, Faraday produced the first known compounds made from carbon and chlorine, hexachloroethane (C2Cl6) and tetrachloroethene (C2Cl4). In 1825, he isolated and identified benzene from the oily residue derived from the production of illuminating gas. Faraday’s discovery of benzene was significant as benzene is one of the most important substances in chemistry. It is immensely useful for both practical purposes, like making new materials; and theoretical purposes, like understanding chemical bonding.
#3 HE WAS THE FIRST TO LIQUEFY CHLORINE AND AMMONIA
John Dalton had theorized that all gases could be liquefied. Michael Faraday provided evidence for this fact by applying pressure to liquefy chlorine gas and ammonia gas for the first time. These were till then believed to be “permanent gases”, or gases incapable of liquefaction. During ammonia liquefaction, Faraday also noted that when he allowed the ammonia to evaporate again, it caused cooling. This discovery showed that mechanical pumps could transform a gas at room temperature into a liquid; this liquid could be evaporated to produce cooling and the resulting gas could be compressed into that liquid again. This cycle is the basis of how modern refrigerators and freezers work.
#4 HE DISCOVERED THE PHENOMENON OF ELECTROMAGNETIC INDUCTION
In 1831, Faraday conducted his most famous experiment. He wrapped two wires around the opposite ends of an iron ring. He plugged one wire into a galvanometer, and watched it as he connected the other wire to a battery. When he connected and disconnected the wire to the battery, a transient current was produced which could be seen in the galvanometer. This induction was due to the change in magnetic flux that occurred when the battery was connected and disconnected. It was thus a manifestation of electromagnetic induction. The phenomenon responsible for Faraday’s experiment is now known as mutual induction. It occurs when change in current in one inductor induces a voltage in another nearby inductor. It is important for being the mechanism by which transformers work.
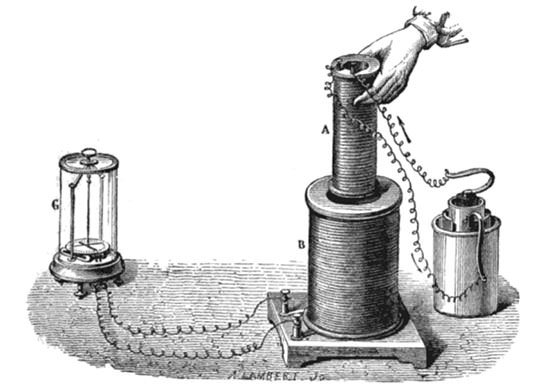
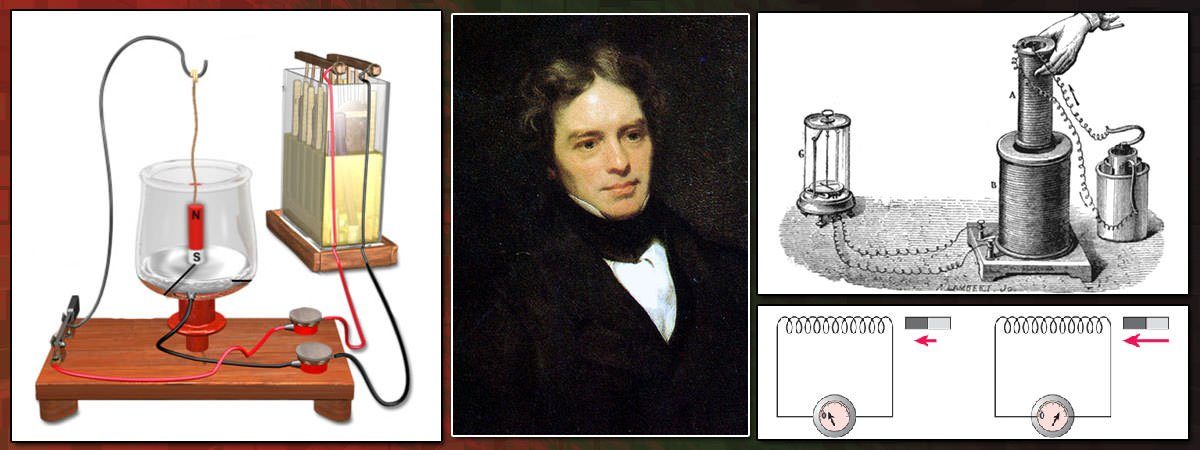
#5 HIS WORK LAID THE BASIS FOR FUTURE DEVELOPMENTS IN ELECTROMAGNETISM
Following his famous experiment, Faraday found other manifestations of electromagnetic induction. He discovered that if a permanent magnet was quickly moved in and out of a coil of wire, a current was induced in the coil. The current also flowed if the loop was moved over a stationary magnet. Faraday’s experiments established that a changing magnetic field produces an electric field. This relation was modeled mathematically by Scottish scientist James Clerk Maxwell as the Maxwell–Faraday equation, one of the four Maxwell equations. Maxwell–Faraday equation plays a fundamental role in classical electromagnetism. It is a generalization of Faraday’s law of induction which predicts how a magnetic field will interact with an electric circuit to produce an electromotive force (EMF). Faraday’s law is the basic operating principle of transformers, inductors, and many types of electrical motors.
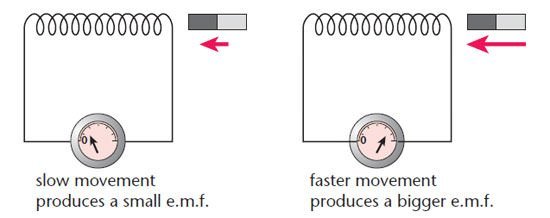
#6 HE INVENTED THE FIRST ELECTROMAGNETIC GENERATOR
Michael Faraday explained electromagnetism through a concept he called lines of force. He discovered that the magnitude of current produced by magnets was proportional to the number of lines of force cut by the conductor in unit time. He invented a device which could produce a steady (DC) current by rotating a copper disc between the poles of a horseshoe magnet. The outside of the disk would cut more lines than the inside, and thus there would be a continuous current produced in the circuit linking the rim to the center. Known as the Faraday disc, this was the first electromagnetic generator, a device which converts mechanical energy to electrical energy. It was also the starting point for modern dynamos, the first electrical generators capable of delivering power for industry.

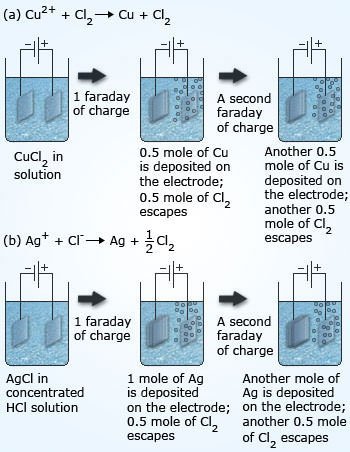
#7 HE FORMULATED FARADAY’S LAWS OF ELECTROLYSIS
In 1832, while conducting investigations into the nature of electricity, Faraday formulated his two laws of electrolysis. The first law states that the amount of a substance deposited on each electrode of an electrolytic cell is directly proportional to the quantity of electricity passed through the cell. The second law states that the quantities of different elements deposited by a given amount of electricity are in the ratio of their chemical equivalent weights. These laws are vital to our understanding of electrode reactions. Michael Faraday was thus one of the key figures in the development of the science of electrochemistry.
#8 HE DISCOVERED THE PRINCIPLE OF ELECTROSTATIC SHIELDING
In 1836, Faraday observed that the excess charge on a charged conductor resides only on its exterior and had no influence on anything enclosed within it. This happens as an external electrical field causes the electric charges within the cage’s conducting material to be distributed such that they cancel the field’s effect in the cage’s interior. Faraday applied this principle to invent the Faraday Cage, which is an enclosure used to block electric fields. Faraday cages are still used for various purposes like to protect people and equipment against lightning strikes and to create dead zones for mobile communications. In 1843, Michael Faraday conducted his famous ice pail experiment to demonstrate this shielding effect. This experiment was the first precise quantitative experiment on electrostatic charge and is still widely used in physics lectures to teach the principles of electrostatics.

#9 HE PROVIDED FIRST EXPERIMENTAL EVIDENCE THAT LINKED ELECTROMAGNETISM AND LIGHT
In 1845, Michael Faraday discovered that the plane of polarization of light was rotated due to a magnetic field and the angle of rotation was proportional to the strength of the magnetic force. This phenomenon is known as the Faraday Effect or Faraday rotation. It occurs in most optically transparent dielectric materials (including liquids) under the influence of magnetic fields. The Faraday Effect is a magneto-optical phenomenon and it provided the first experimental evidence that electromagnetism and light are related. Later, in 1864, James Maxwell established that light is an electromagnetic wave.

#10 HE DEMONSTRATED DIAMAGNETISM AS A PROPERTY OF ALL MATTER
Diamagnetic materials are those which create an induced magnetic field in a direction opposite to an externally applied magnetic field, and are repelled by the applied magnetic field. Paramagnetic materials behave oppositely and are attracted by an externally applied magnetic field. Diamagnetic behaviour was first observed in certain materials in 1778. Michael Faraday demonstrated that Diamagnetism was a property exhibited by all substances (in either a diamagnetic or paramagnetic way). Diamagnetism in materials, induced by very strong modern magnets, can be used to produce levitation.