Antoine-Laurent de Lavoisier (1743 – 1794) was a French chemist who is most famous for changing chemistry from a qualitative to a quantitative science and for discovering the role of oxygen in combustion. Prior to Lavoisier, the dominant theory to explain combustion was the phlogiston theory, which was ultimately disproved by his work. Lavoisier made many other important contributions to the field of chemistry which include establishing water as a compound of hydrogen and oxygen; discovering that sulfur is an element and that diamond is a form of carbon; establishing law of conservation of mass in chemistry; and co-authoring the first modern system of chemical nomenclature. Lavoisier had a huge influence on the history of chemistry and he is renowned as the “father of modern chemistry”. Know more about the inventions, discoveries and other accomplishments of Antoine Lavoisier through his 10 major contributions.
#1 HE DISPROVED THE PREVALENT PHLOGISTON THEORY OF COMBUSTION
In early 18th century, German scientist Georg Ernst Stahl proposed the theory of phlogiston to explain combustion, which became widely accepted. According to it, every combustible substance contained a universal component of fire called phlogiston. This substance was released during combustion, respiration and calcination; and absorbed when these processes were reversed. However, when metals were heated, the resulting oxide weighed more than the original metal. But, according to Stahl’s hypothesis they should have weighed less as the metal had lost the phlogiston component. Proponents of the theory even suggested that phlogiston might have a negative weight. In 1772, Antoine Lavoisier conducted his first experiments on combustion. He reported that when Phosphorus and Sulphur are burned, they gained weight by combining with air and that the products were acidic. Lavoisier worked on combustion over the next fifteen years and his work ultimately disproved the phlogiston theory of combustion.

#2 He PUT FORWARD THE OXYGEN THEORY OF COMBUSTION
Antoine Lavoisier understood that elements combined with something in the air leading to gain in their weight. In 1774, English scientist Joseph Priestley isolated a component of air by heating mercury calx (oxide). He believed it to be a pure version of air as it supported respiration and combustion in an enhanced way. Priestly called it dephlogisticated air, believing its unusual properties were caused by the absence of phlogiston. When he informed Lavoisier of his discovery, Lavoisier repeated the experiment with mercury and other metal oxides. He concluded that air had two components: one that combined with the metal and supported respiration; and the other that did not support either combustion or respiration. In 1778, Lavoisier put forward his new theory of combustion by which combustion was the reaction of a metal or an organic substance with that part of common air he termed “eminently respirable”. The following year, he coined the name “oxygen” for it, from the Greek words meaning “acid generator”. Lavoisier’s discovery of the role oxygen plays in combustion is considered one of his major achievements.
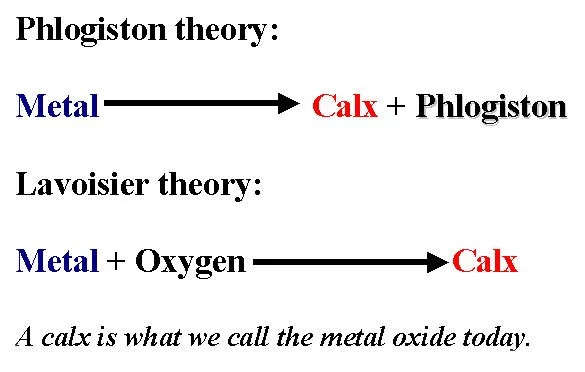
#3 HE Co-ESTABLISHED THAT WATER WAS A COMPOUND AND NOT AN ELEMENT
In 1783, Antoine Lavoisier coined the name “hydrogen” for the gas which Henry Cavendish had recognized as a new element in 1766. Cavendish had called the gas inflammable air. In cooperation with French mathematician Pierre Simon de Laplace, Lavoisier began a series of experiments on the composition of water in 1783. The two burned jets of hydrogen and oxygen in a bell jar over mercury to obtain “water in a very pure state”. The quantitative results were good enough to support the contention that water was not an element but a compound of two gases, hydrogen and oxygen. This was a remarkable discovery as everyone had considered water to be an element from the time of Aristotle who included it in his four elements; over 2,000 years ago. The interpretation of water as compound also explained the inflammable air (hydrogen) generated from dissolving metals in acids and the reduction of oxides by the inflammable air.

#4 HE CO-DISCOVERED THAT RESPIRATION IS A FORM OF COMBUSTION
In 1782–83, along with Pierre Simon de Laplace, Lavoisier conducted experiments in the area of respiration physiology. The pair used a calorimeter to measure the amount of heat given off by a guinea pig in a measured interval of time. They also measured the amount of carbon dioxide (then called fixed air) given off by the guinea pig in this same interval. They found that a similar amount of heat was produced when sufficient carbon was burned in the ice calorimeter to produce the same amount of carbon dioxide as that which the guinea pig exhaled. From this, Lavoisier and Laplace concluded that respiration was similar to slow combustion. This enables the living animal to maintain its body temperature above that of its surroundings. The experiment accounted for the puzzling phenomenon of animal heat. The pioneering work of Lavoisier and Laplace in the field served to inspire similar research on physiological processes for generations to come.
#5 HE CO-AUTHORED THE FIRST MODERN SYSTEM OF CHEMICAL NOMENCLATURE
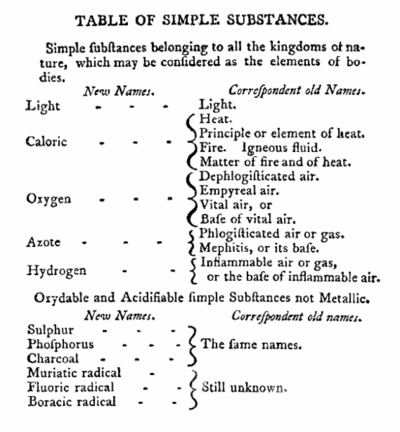
Together with French chemists Louis-Bernard Guyton, Claude Louis Berthollet and Antoine Francois, Lavoisier published in 1787 a work titled Méthode de nomenclature chimique (Method of Chemical Nomenclature). This was the first proper system of chemical nomenclature, i.e. a system of names describing the structure of chemical compounds. It was based on three general principles: substances should have one fixed name; it should reflect composition when known; and it should generally be chosen from Greek or Latin roots. 55 substances which could not be decomposed into simpler substances by any known chemical means were listed as elements in the publication. The list was not totally accurate and included light and caloric (matter of heat). The acids, which were recognized as compounds in the system, were given names according to the degree of oxygenation, like nitric and nitrous acids. The “ic” termination indicated acids with a higher proportion of oxygen than those with the “ous” ending. The new nomenclature spread throughout the world and became common use in the field of chemistry.
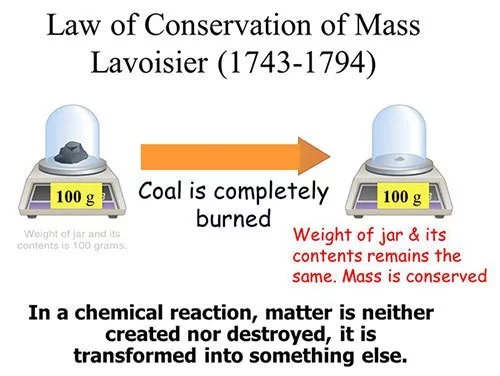
#6 HE IS CREDITED WITH ESTABLISHING MASS CONSERVATION IN CHEMICAL REACTIONS
In 1778, Lavoisier found that when mercury oxide is heated its weight decreases; and the oxygen released has the same weight as the weight lost by mercury oxide. After carrying out work with a number of different substances, he concluded that this was due to the law of conservation of mass, which states that the total mass of matter is the same at the end as at the beginning of every chemical change. Though the principle of conservation of matter had been stated by several people earlier, Lavoisier illustrated it with experiments and employed a criteria for conservation: the total mass of the products must come from the mass of the reactants. The law of conservation of mass became established only after Lavoisier’s efforts and many credit him for discovering mass conservation in chemical reactions. In fact in France, the law is still taught as Lavoisier’s Law.
#7 HE DISCOVERED THAT SULFUR IS AN ELEMENT AND THAT DIAMOND IS A FORM OF CARBON
In 1772, Antoine Lavoisier and other chemists placed a diamond in a glass jar and focused sun’s rays on it with a giant magnifying glass. The diamond burned and disappeared. Lavoisier found that whether diamond or charcoal was burnt, neither produced any water and both released the same amount of carbon dioxide per gram. He thus discovered that diamond is a crystalline form of carbon introducing the possibility of allotropy in chemical elements. In 1777, Lavoisier carried out extensive experiments involving sulfur and found that it could not be broken down into any simpler substances. He thus became the first person to establish that sulfur was an element and not a compound. In 1787, Lavoisier suspected that silica might be an oxide of a fundamental chemical element thus predicting the existence of silicon.
#8 HE WROTE THE FIRST MODERN TEXTBOOK ON CHEMISTRY
In 1789, Antoine Lavoisier published his most famous work Traité élémentaire de chimie (Elementary Treatise of Chemistry). The book established Lavoisier’s oxygen theory of combustion and denied the existence of phlogiston. It defined an element as a single substance that can’t be broken down by chemical analysis and from which all chemical compounds are formed. It contained a list of elements, which formed the basis for the modern list of elements. It explained the influence of heat on chemical reactions; the nature of gases; the reactions of acids and bases to form salts; and the apparatus used to perform chemical experiments. It also presented a unified view of new theories of chemistry and contained a clear statement of the law of conservation of mass. Elementary Treatise is regarded as the first modern textbook on the subject of Chemistry. It went on to be hugely influential and remains a classic in the history of science.

#9 LAVOISIER IS CONSIDERED THE FATHER OF MODERN CHEMISTRY
Lavoisier is most famous for changing chemistry from a qualitative to a quantitative science. He performed some of the first truly quantitative chemical experiments. He carefully weighed the reactants and products of a chemical reaction in a sealed glass vessel so that no gases could escape, which was a crucial step in the advancement of chemistry. Lavoisier is considered a pioneer of stoichiometry, branch of chemistry concerned with calculation of relative quantities of reactants and products in chemical reactions. He was responsible for the construction of the gasometer, a large container in which natural gas is stored. It enabled him to weigh the gas in a pneumatic trough with the precision he required. He also established the consistent use of the chemical balance, a device used to measure weight. The work of Lavoisier raised the level of chemistry leading to it becoming as important as physics and mathematics. For all his accomplishments in the field, Antoine Lavoisier is widely regarded as the “father of modern chemistry”.

#10 HE CONTRIBUTED IN THE ADOPTION OF THE METRIC SYSTEM
Apart from his contributions to science, Antoine Lavoisier also did a lot of work as a humanitarian. In 1765, he submitted an essay on improving urban street lighting to the French Academy of Sciences for which he was awarded a gold medal by King Louis XV. He worked on projects to purify the water from the Seine; to improve air quality and study health risks associated with gunpowder’s effect on the air; to improve living conditions of prisoners; to reform the French monetary and taxation system to help the peasants; and to improve the agricultural yields in the Sologne. In 1791, Lavoisier chaired the commission set up to establish a uniform metric system. Other members of the committee including the well-known mathematicians Pierre-Simon Laplace and Adrien-Marie Legendre. The new system of uniform weights and measures was adopted by the Convention on 1 August 1793.

